Delee
por Liza Velarde
Aviso Importante: Esta iniciativa puede encontrarse en fase de idea. Será responsabilidad de las personas con interés en hacer una simulación de inversión de carácter informativo exclusivamente, el darle seguimiento al emprendedor, hacer el correspondiente análisis legal y financiero, y de llegar a un acuerdo entre las partes, concretarán la intensión de participación realizando la aportación económica directamente en la cuenta bancaria de la iniciativa. Las aportaciones económicas para esta iniciativa no se realizan a través de Inixar. Inixar no es una institución tecnológica financiera, sólo facilita el acercamiento entre personas.
Creado por

Liza Velarde
Sobre este Proyecto
Welcome to the next generation of cancer blood tests
Delee has developed a patent pending technology capable of efficiently isolating and analyzing circulating tumor cells from blood, which could dramatically increase the information that physicians have for detecting cancer at early stages and monitoring the applied treatments’ effectiveness.
CytoCatch™ Isolation Platform and Imaging System
Our technology will enable a broad range of clinical applications in oncology, including:



Benefits of our technology
The early detection of cancer will be translated into invaluable benefits for patients by greatly increasing their chances of defeating cancer. Furthermore, monitoring the treatments’ effectiveness could also significantly increase the odds of defeating cancer by applying the most effective treatment for each patient throughout the course of the disease, while reducing the incurred costs and the negative side effects caused by drugs that wouldn’t be effective for a particular patient.
For what types of cancer?
Our technology can be used for a wide variety of cancer types. There is scientific evidence that indicates the feasibility of developing CTC assays to enable clinical applications in various types of cancer, including prostate, breast, colorectal, lung, cervical, skin, and ovarian cancer, just to name a few. Over 40% of the new cancer cases and a third of the defunctions registered worldwide are attributed to prostate, breast, colorectal, and lung cancer.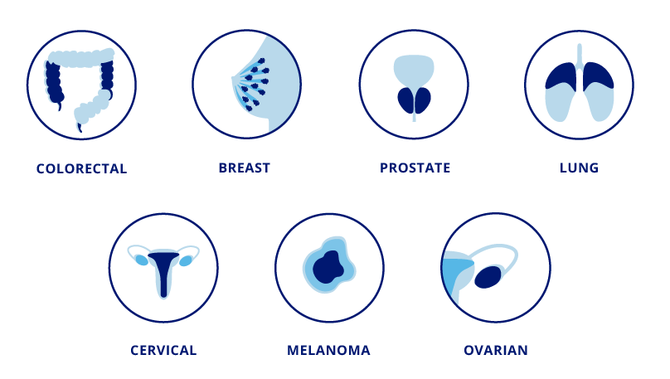
Our technology
A complete sample-to-answer solution that will help physicians make information-driven decisions
The blood sample (extracted by conventional venipuncture, the collection method that is usually used for laboratory testing) along with several reagents are loaded on the CytoCatch™ isolation platform, which automatically performs the necessary steps to prepare and process the sample, capturing the CTCs contained in it. The unit has an outstanding performance, it has the sensitivity to isolate a single CTC from a background of 56 billion blood cells, and has recovery rates above 96% when processing 10 mL blood samples spiked with tumor cells from prostate, breast, and colorectal cancer cell lines, meaning that the platform recovers at least 96 out of 100 tumor cells spiked into the sample.
Once captured, the CytoCatch™ isolation platform executes an automated protocol to stain the collected cells with fluorescent antibodies for their further analysis with the CytoCatch™ imaging system, which possesses special routines and machine learning algorithms that analyze the captured cells based on their morphology and the expression of specific markers. The fact that all these processes are fully automated increases the reliability and reproducibility of the assay by preventing human errors and cell loss due to manual steps.
Furthermore, the collected cells are compatible with traditional molecular biology techniques and next generation sequencing technologies, enabling the performance of molecular analyses to assess the genetic characteristics of the captured CTCs. Finally, the treating physician will get a report with the corresponding results.
Workflow






Traction
Pre-orders worth a potential value over $2.5 million USDWe’ve gotten great traction since the pre-commercial launch of our technology. To date, pre-orders worth a potential value of over $2.5 million USD have been secured from research centers of various hospitals, including the Stanford University Medical Center and the “Dr. José Eleuterio González” University Hospital. These institutions will use our technology as a research use only device, for which FDA clearance is not required.

IP and peer-reviewed articles
We have three patent pending applications that protect different aspects of our technology and published two peer-reviewed articles in Nature's Scientific Reports.


Customers
We'll start sales of our technology as a research use only device by Q4 of 2022, for which FDA clearance is not required, being pharmaceutical companies and research centers our main customers. Once our technology obtains FDA clearance, it will be commercialized as an in vitro diagnostic medical device for its use in hospitals and laboratories.
Business model
We will implement the razor and blades business model, obtaining revenue by selling the CytoCatch™ Isolation Platform and Imaging System, and recurring revenue by selling the necessary reagents and consumables to perform each test.

Market
The CTC market is massive and will continue to grow over timeAccording to a report published by Grand View Research, in 2019, the global circulating tumor cell market was valued at $8.9B USD, and it’s expected to reach a $23.9B USD valuation by 2027. This valuation is mainly due to the ongoing research that is carried out by numerous research centers to validate the use of these cells as a biomarker for different clinical applications.

Once our technology obtains FDA clearance, it will be commercialized as an in vitro diagnostic medical device for its use in hospitals and laboratories, for early cancer detection and monitoring the applied treatments’ effectiveness, accessing a total addressable market of $543B USD.
Competition
The new standard for capturing and analyzing CTCs
Most of the blood tests employed as auxiliaries in the diagnosis of cancer and monitoring of the applied treatments’ effectiveness measure protein tumor markers levels, such as PSA, CA-125, and AFP. However, there are only a few protein tumor markers that are associated with a particular cancer and are clinically useful; most types of cancer have not been linked to an increase in the levels of a particular protein tumor marker. Furthermore, these types of tests have a poor sensitivity and specificity, meaning that these markers may be elevated in people that do not have cancer and that not every person with a particular type of cancer will have an elevated level of the corresponding tumor marker. Taking the PSA test as an example, which measures the amount of PSA in blood and is used to screen for prostate cancer, approximately 79% of men with increased levels of PSA do not have prostate cancer, whereas 9% of the men with normal levels of PSA may have prostate cancer.
The isolation and analysis of CTCs is a relatively new practice, and physicians are starting to recognize all its potential benefits. Most of the current CTCs technologies, including the CellSearch® System, which currently is considered the gold standard, rely on the existence of specific proteins on the tumor cells’ membranes in order to capture them. However, CTCs are incredibly heterogeneous; when entering the bloodstream, they undergo a biological process that downregulates these proteins, limiting the efficiency with which these cells are captured and thereby losing valuable information. Our technology changes the norm by isolating CTCs irrespective of the proteins expressed in their membranes, allowing us to capture tumor cells that other technologies can’t.

Milestones
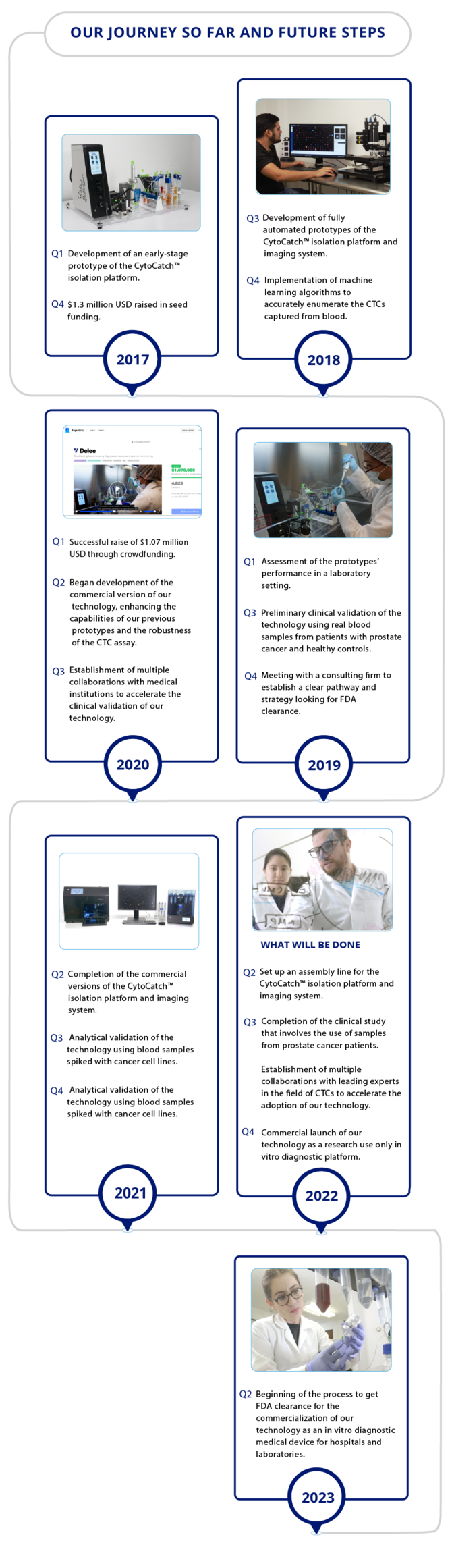
Funding
Delee has raised over $2.6M USDWe are backed by Y Combinator and StartX, two of the most important startup accelerators in the world. Delee is also funded by Emles venture partners and raised over $1.4M USD on equity crowdfunding from more than 5250 investors.

Team
Founders

Directors and advisors